تُعد انتانات جرثومة المعدة (الملوية البوابية Helicobacter pylori) من الانتانات الشائعة في جميع أنحاء العالم وسبباً مهماً للإصابة بالقرحة الهضمية وسرطان المعدة.
مقدمة عن جرثومة المعدة (Helicobacter pylori)
عُرفت جرثومية المعدة واسمها العلمي (الحلزونية البوابية Helicobacter pylori) كعامل ممرض رئيسي للبشرية لما يقرب من أربعة عقود، وعلى الرغم من تأثير علاج الأفراد المصابين وانخفاض انتقال الجرثومة في المجتمعات التي تحسنت فيها مستويات المعيشة الاجتماعية والاقتصادية فإنها لا تزال أكثر أسباب الأمراض الجرثومية البشرية شيوعاً إذ تصيب ربما نصف سكان العالم، ونتيجة لذلك لا تزال جرثومة المعدة (الحلزونية البوابية) سبباً رئيسياً للمراضة والوفيات في جميع أنحاء العالم.(1)
عادة ما تستمر عدوى جرثومة المعدة (الحلزونية البوابية) مدى الحياة إذا لم تُعالج بالصادات الحيوية أو قد يحدث استئصال ذاتي لها كنتيجة للعدوى طويلة الأمد التي تؤدي إلى ضمور الغشاء المخاطي المعدي وتغيره مع فقدان حموضة المعدة، وهناك اختلافات في فوعة سلالات جرثومة المعدة على مستوى العالم، وقد يؤدي التفاعل بين العائل المضيف والعوامل البيئية إلى اختلافات في التعبير عن المرض.(1)
صفات جرثومة المعدة (Helicobacter pylori) وآلية تأثيرها
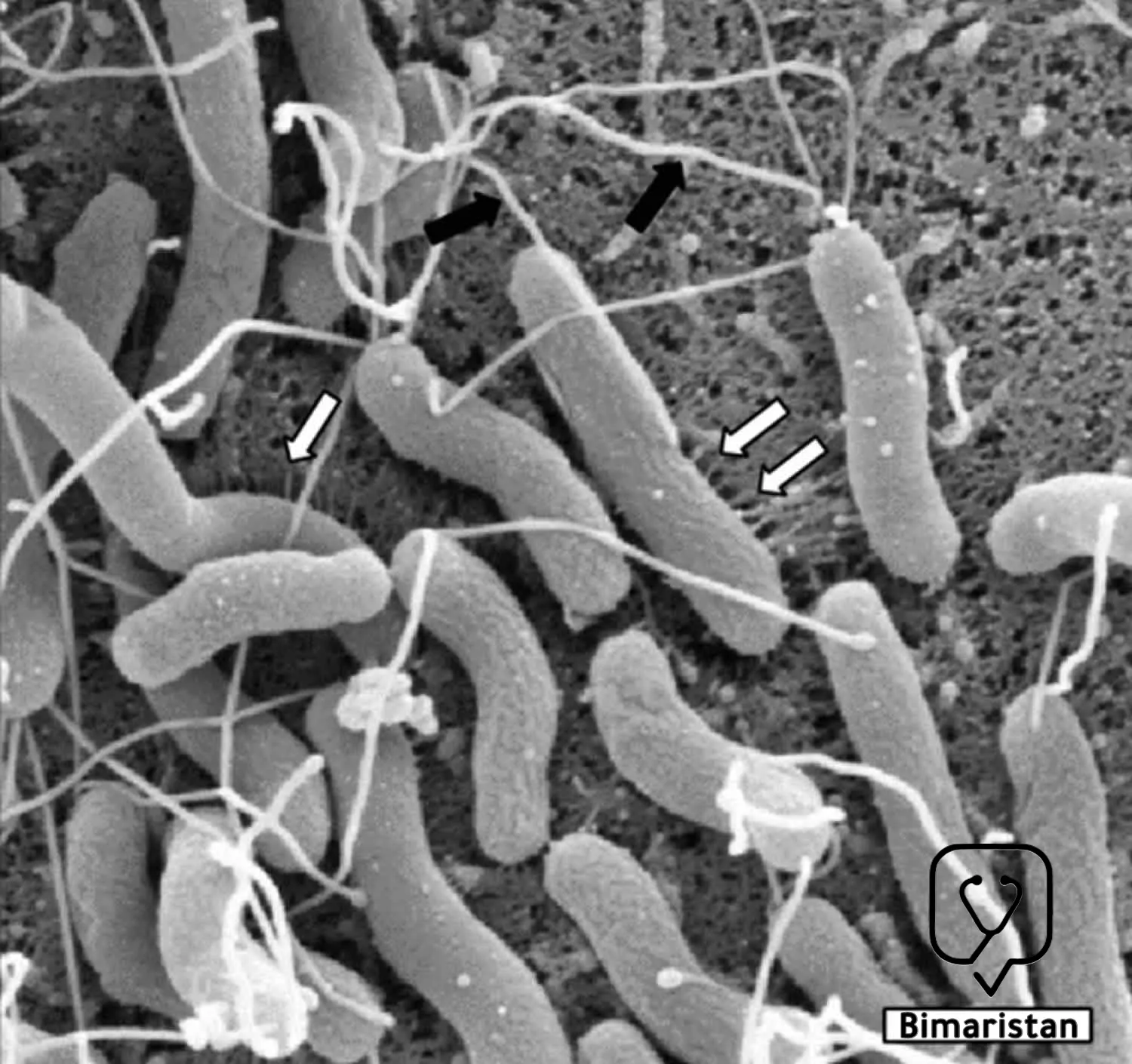
تعد جرثومة المعدة (الحلزونية البوابية) بكتيريا سالبة الغرام بطيئة النمو قليلة الألفة للهواء، يبلغ عرضها 0.5-0.8 ميكرون ولها عدة أسواط في أحد طرفيها، وتتواجد جرثومة المعدة بشكلين كعصيات حلزونية متحركة ومكورات غير متحركة، وتتميز جرثومة المعدة بالقدرة على التغلب على المحتوى الحمضي المرتفع في المعدة عن طريق عوامل الفوعة التي تعزز استعمار الغشاء الظهاري المعدة وتحريض تلفه.(2)
لا تغزو جرثومة المعدة (الحلزونية البوابية) الغشاء المخاطي ولكنها تعيش بالتصاقها بالخلايا الظهارية المخاطية السطحية يُطلق استجابات مناعية ملحوظة، فهي تعمل على إثارة استجابة التهابية مزمنة تشمل خلايا الدم البيضاء المعتدلة تليها الخلايا الليمفاوية التائية والبائية وخلايا البلازما والبالعات والتي تتسبب مع بعضها بإحداث تلف الغشاء المخاطي للمعدة من خلال أنشطتها المناعية. وهناك استجابة الأجسام المضادة تجاه عديد السكريات الشحمي في جرثومة المعدة وهي بشكل رئيسي أضداد IgG وهناك أيضاً أضداد IgA و IgM لكنها أقل نوعية.(2)
انتشار عدوى جرثومة المعدة (Helicobacter pylori)
يزداد انتشار عدوى جرثومة المعدة (الحلزونية البوابية) مع تقدم العمر وقد تم اعتبار العرق بأنه عامل خطر، ولكن من المرجح أن يرتبط الانتشار ارتباطاً وثيقاً بالحالة الاجتماعية والاقتصادية أو الممارسات التي قد تزيد من خطر انتقال العدوى أكثر من ارتباطه بالوراثة.(1)
تُشير التقارير إلى أن معدلات انتشار جرثومة المعدة تتراوح بين 20-50% في السكان البالغين في الدول المتقدمة ولكن الانتشار أكثر بكثير في البلدان النامية فقد يصل إلى 90% في بعضها، كما أن الانتشار أعلى في المناطق ذات الظروف الاجتماعية والاقتصادية المنخفضة والظروف الصحية السيئة وفي المناطق الريفية، يبدو أن الحالة الاجتماعية والاقتصادية للأسرة أثناء الطفولة هي العلامة الرئيسية للعدوى بجرثومة المعدة.(2)
إن الانخفاض في معدل انتشار جرثومة المعدة (الحلزونية البوابية) له علاقة بتحسن إجراءات النظافة العامة للسكان والصرف الصحي أكثر من علاج كل حالة على حدة، ويبدو أن معدل انتشار عدوى جرثومة المعدة مستقراً في البلدان التي لم تتحسن فيها/تتدهور فيها معايير الصحة والنظافة العامة ومن غير المرجح أن تنخفض بشكل كبير حتى يحدث التحسن المطلوب.(1)
طرق انتقال عدوى جرثومة المعدة (Helicobacter pylori)
يمكن أن يحدث انتقال جرثومة المعدة (الحلزونية البوابية) بالطريقين العمودي أو الأفقي، ففي الانتقال العمودي تنتقل جرثومة المعدة من الآباء إلى الأطفال من خلال الطريق الفموي-الفموي عبر طريق اللعاب المصاب بالعدوى، بينما في الانتقال الأفقي يصاب الناس بالجرثومة من خلال التلوث البيئي أو التفاعلات الاجتماعية.(3)
أحد أكثر طرق انتقال جرثومة المعدة (الحلزونية البوابية) شيوعاً هو الطريق البرازي-الفموي، فقد تم إثباته بشكل غير مباشر من نتائج دراسات أمريكا الجنوبية التي أظهرت أن إمدادات المياه التي قد تحتوي على جرثومة المعدة إضافة إلى تناول أنواع الخضار النيئة الملوثة ببراز الإنسان هي عوامل خطر كبيرة للعدوى.(3)
يعد الطريق الفموي-المعدي وسيلة انتقال مهمة خاصة في البيئات غير الصحية، فقد تم اكتشاف جرثومة المعدة في ظهر اللسان وجيوب اللثة باستخدام طريقة تفاعل البوليميراز المتسلسل (PCR)، كما كانت هناك علاقة مهمة بين إيجابية عينات اللسان والأظافر، وفقاً لهذه النتائج يقترح بعض الباحثين أن جرثومة المعدة يمكن أن تنتقل عن طريق اليدين وأن وضع الإصبع في الفم يمكن أن يكون وسيلة مهمة لانتقال جرثومة المعدة.(3)
الأعراض السريرية لعدوى جرثومة المعدة (Helicobacter pylori)
يتم اكتساب عدوى جرثومة المعدة (الحلزونية البوابية) عادة في عمر 10 سنوات وتبقى حوالي 85% من الحالات بدون أعراض لفترات طويلة، وعند ظهور الأعراض السريرية فهي غير نوعية وبعضها يُعزى إلى المضاعفات. وقد حاولت الدراسات التحليلية تحري وجود ارتباط محتمل بين عدوى جرثومة المعدة والأعراض السريرية وتبين أنه لا علاقة للعدوى بحدوث الإقياء أو الإسهال أو الغازات أو آلام البطن المزمنة أو رائحة الفم الكريهة أو القلس أو الإمساك أو الغثيان، ولكن وجدت ارتباطاً إحصائياً بحدوث ألم شرسوفي.(4)
الأمراض المرتبطة بعدوى جرثومة المعدة (Helicobacter pylori)
لقد ثبت أن بكتيريا المعدة تسب العديد من الأمراض بما في ذلك:
التهاب المعدة
يمكن أن تؤدي بكتيريا الحلزونية البوابية إلى الإصابة بالتهاب المعدة الحاد أو المزمن، وعادة ما تتميز الإصابة الحادة بأنها مرض خفيف عابر يتظاهر بألم شرسوفي وغثيان ومظاهر نسيجية لالتهاب المعدة بالعدلات ونقص حموضة المعدة العابر. غالباً ما يظهر التهاب المعدة المزمن في شكل التهاب معدي سطحي غير ضموري مزمن فعال مع تلف بؤري للخلايا الظهارية المبطنة للمعدة، وعادة ما يكون بدون أعراض على الرغم من أنه قد يكون مرتبطا بمرض القرحة الهضمية، وقد يتطور التهاب المعدة الضموري المزمن من الشكل غير الضموري في نسبة أقل من مرضى التهاب المعدة.(2)
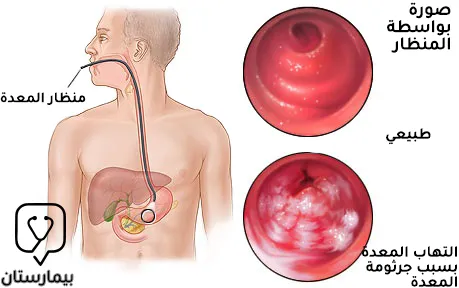
مرض القرحة الهضمية
يكون لدى المصاب بعدوى بكتيريا الحلزونية البوابية خطر لحدوث مرض القرحة الهضمية على مدى الحياة بنسبة 10-20% وهو أعلى بـ 3-4 أضعاف على الأقل مقارنة بالأشخاص غير المصابين، ويمكن تشخيص بكتيريا الحلزونية البوابية لدى 90-100% من مرضى قرحة الاثني عشري ولدى 60-100% من مرضى قرحة المعدة. كما يمكن أن يتطور التهاب المعدة الناجم عن جرثومة المعدة إلى تقرح في الغشاء المخاطي نتيجة حدوث موت الخلايا الظهارية المبرمج وما يتلوه من تأذي الحاجز الوقائي المخاطي مما يعرض الغشاء المخاطي للمعدة إلى هجوم مباشر من حمض اللومينال والبيبسين.(2)
يحدث فرط إفراز الحمض في القرحة الاثني عشرية دائماً تقريباً بسبب عدوى جرثومة المعدة لأن الإفراز يعود إلى طبيعته بعد القضاء عليها. يقلل التهاب غار المعدة السائد في قرحة الاثني عشري من عدد الخلايا المنتجة للسوماتوستاتين في الغار، هذا يؤدي إلى انخفاض في إنتاج السوماتوستاتين وانخفاض تثبيط تحرير الغاسترين المتواسط بالسوماتوستاتين من الخلايا المعدة وبالتالي يتطور فرط غاسترين الدم. وقد تؤدي زيادة إفراز الحمض ذاتياً إلى زيادة خطر الإصابة بتقرح الاثني عشر أو قد يؤدي إلى حدوث حؤول معدي في الاثني عشر، والذي يصبح مستعمراً بواسطة جرثومة المعدة، ثم يحدث التهاب الاثني عشر ويتقرح في النهاية. ويؤدي الاستئصال الناجح لجرثومة المعدة إلى شفاء مرض القرحة الهضمية ويقلل من تكرار حدوث القرحة.(2)
سرطان المعدة الغدي
صنفت منظمة الصحة العالمية جرثومة المعدة كمسرطن من المجموعة 1 يؤدي إلى سرطان غدي معدي بناء على الأدلة القاطعة، وتشمل المناطق التي يزداد فيها معدل الإصابة بسرطان المعدة التالي للعدوى بجرثومة المعدة اليابان والشرق الأوسط وجنوب شرق آسيا والبحر الأبيض المتوسط وأوروبا الشرقية وأمريكا الوسطى وأمريكا الجنوبية.(5)
يحدث سرطان المعدة ضمن سلسلة من الأحداث التي تبدأ بالتهاب المعدة السطحي المزمن الناجم عن جرثومة المعدة وتتطور نحو التهاب المعدة الضموري، ثم الحؤول المعوي وخلل التنسج وفي النهاية سرطان المعدة، ويستغرق هذا التسلسل عقوداً ليكتمل.(2)
على الرغم من أنه يُعتقد الآن أن جرثومة المعدة مسؤولة عن 80% أو أكثر من سرطانات المعدة إلا أن 3% فقط من المرضى المصابين يتطورون إلى سرطان المعدة، وبالتالي فإن عدوى جرثومة المعدة وحدها لا يكفي بشكل عام للتسبب في سرطان المعدة وإنما عوامل متعددة من العوامل البكتيرية والظروف البيئية والاستجابة المناعية للمضيف وعوامل وراثية أخرى هي المسؤولة.(2)
الليمفوما المرتبطة بالغشاء المخاطي المعدي (MALT)
تُعتبر المسببات الجزيئية لليمفوما المرتبطة بالغشاء المخاطي المعدي (MALT) غير مفهومة بشكل كامل ولكن يبدو أنها تتضمن عوامل جرثومية نوعية من جرثومة المعدة إضافة إلى العوامل الوراثية للمصاب مثل محفزات السيتوكينات الالتهابية، ويُعتقد أن عدوى جرثومة المعدة تؤدي إلى تكوين خلايا تائية تفاعلية والتي تتسبب بعد ذلك في تكاثر الخلايا البائية متعددة النسيلة، وبمرور الوقت يظهر ورم الخلايا البائية وحيدة النسيلة في الخلايا البائية المتكاثرة كنتيجة لتراكم الطفرات في الجينات المنظمة للنمو.(2)
عسر الهضم الوظيفي
إن معدل انتشار جرثومة المعدة مرتفع بشكل عام في المرضى الذين يعانون من عسر الهضم بغض النظر عن المجموعة الفرعية، وقد تم إثبات تأثير جرثومة المعدة في التسبب في عسر الهضم القرحي ولكن هناك آراء مخالفة حول الدور الذي تلعبه في التسبب في عسر الهضم الوظيفي، بينما أظهرت بعض الدراسات ارتباطاً بين عدوى جرثومة المعدة والتشخيص السريري لعسر الهضم الوظيفي.(2)
أمراض خارج الجهاز الهضمي
هناك علاقة بين حالات خارج الجهاز الهضمي وجرثومة الملوية البوابية، فقد لوحظ ارتباط مع مرض الشريان التاجي نتيجة وجود عوامل خطر مشتركة كالفقر والتغذية غير المثالية، كما أن هناك ارتبلط لفقر الدم بعوز الحديد غيرالمفسر ونقص الصفيحات المناعي بعدوى جرثومة المعدة وعلى الرغم من أن الآلية المرضية غير مفهومة جيداً إلا أن التقارير عن العلاج الناجح لعدوى جرثومة الملوية البوابية التي تؤدي إلى زيادة مستوى الخضاب أو ارتفاع عدد الصفائح الدموية تشير إلى وجود علاقات سببية بينهما.(5)
تشخيص عدوى جرثومة المعدة (Helicobacter pylori)
الإجراءات التشخيصية الباضعة
الخزعة النسيجية: يتم الحصول على الخزعات من غار وجسم المعدة ويتم تلوين مستويات متعددة من كل خزعة بشكل روتيني بالهيماتوكسيلين والأيوزين إضافة إلى صبغات خاصة أخرى، وتُستخدم صبغة الهيماتوكسيلين والأيوزين لتحديد التهاب المعدة المزمن أو المزمن الفعال ولكن يمكن أيضاً أن تظهر جرثومة الملوية البوابية إذا كانت موجودة بأعداد كبيرة، ويمكن اكتشاف أعداد صغيرة من جرثومة المعدة بشكل أفضل من خلال الصبغات الخاصة. من المزايا المهمة لدراسة الخزعة النسيجية أنه يمكن فحص أقسام من الخزعات في المستقبل، وتشمل عيوبها انها تعتمد بشكل كبير على خبرة الفاحص وتحتاج وقتاً طويلاً نسبياً لظهور النتيجة.(2)
اختبار اليورياز السريع: يتم استخدام إنزيم اليورياز الذي تنتجه حرثومة المعدة في إجراء هذا الاختبار إذ يتم وضع خزعة المعدة في وسط يحتوي على اليوريا ومشعر PH، ويقوم إنزيم اليورياز بتفكيك اليوريا لإنتاج الأمونيا التي تزيد من درجة الحموضة في الوسط مما يؤدي إلى تغيير اللون. يتميز هذا الاختبار بنوعية وحساسية أكبر من 90% ولكن تظهر نتائج إيجابية كاذبة، ويمكن إجراء الاختبار وقراءته في غضون 1-24 ساعة، ويتميز هذا الاختبار بسرعته وبساطته ورخص ثمنه مقارنة بالخزعة ولكن لا يمكن استخدامه لتقييم التهاب المعدة.(2)
الزرع الجرثومي: يُجرى الزرع ضمن ظروف صارمة فيجب نقل خزعة التنظير إلى المختبر عند درجة حرارة 4 مئوية خلال 24 ساعة أو عند درجة -70 مئوية لفترة أطول، وبعد زرع العينة في وسط الزرع يتم فحص الأطباق لمدة 10 أيام تقريباً، وعادة ما يتم أخذ خزعات متعددة من غار وجسم المعدة بسبب الطبيعة البؤرية للآفات الالتهابية التي تنتجها جرثومة المعدة. تبلغ نوعية الزرع 100% بينما الحساسية أقل بقليل، والميزة الرئيسية للزرع الجرثومي أنه يمكن الحصول على نمو نقي لجرثومة المعدة لإجراء دراسات تفصيلية كالحساسية للصادات الحيوية عندما يكون هناك فشل في أدوية الخط الثاني، وبتحديد نوع السلالة الجرثومية، ولإجراء الدراسات الجينية وما إلى ذلك، بينما تشمل عيوب الزرع أنه يتطلب وقت طويل لظهور النتائج والتكلفة العالية والظروف الصارمة اللازمة لنقل العينة إلى المختبر.(2)
تفاعل سسلسلة البوليميراز (PCR): يمكن الحصول على عينة من المعدة نتيجة لتطوير جهاز اختبار الكبسولة الخيطية من أجل PCR (دون الحاجة إلى خزعة المعدة بالتنظير كما كان سابقاً)، يتميز هذا الاختبار بحساسية ونوعية تفوق 90% ويمكن استخدامه لتحليل الأنماط الجينية البكتيرية ودراسة نمط مقاومة الصادات الحيوية وانتقال جرثومة المعدة داخل العائلات والمجتمع، ولكن العيب الرئيسي لهذا الاختبار أنه باهظ الثمن ويتطلب خبرة فنية لتطبيقه.(2)
المقايسة المناعية لإنزيم الحمض النووي: تعتمد على اختبار الـ ELISA باستخدام حجرات دقيقة مغلفة، وهذه الطريقة أسرع من PCR القياسي ويمكن الحصول على النتيجة في غضون ساعات قليلة.(2)
التهجين التألقي (FISH): وهو عبارة عن اختبار جزيئي آخر لتشخيص جرثومة المعدة يُفيد بشكل خاص في الكشف عن مقاومة حساسية جرثومة المعدة للصاد الحيوي كلاريثروميسين.(2)
الإجراءات التشخيصية غير الباضعة
الاختبارات المصلية: وتسبب العدوى المزمنة بجرثومة المعدة استجابة منتشرة للأضداد يمكن قياسها كمياً بالتقنيات المصلية كالمقايسة المناعية المرتبطة بالإنزيم (ELISA)، على الرغم من إمكانية إجراء اختبارات أضداد IgG و IgA و IgM إلا أن اختبار أضداد IgG هو الوحيد الموثوق به، ويتم استخدام مصل الدم أو البلازما ويمكن استخدام الدم الكامل مؤخراً.(2)
تستخدم الاختبارات المصلية بشكل شائع في دراسات انتشار جرثومة المعدة وذلك نظراً لسهولتها توفرها والقدرة على تحمل تكاليفها وبساطتها، ولكن عيبها الرئيسي هو قوتها التمييزية الضعيفة بين العدوى الحالية والسابقة لأنها قد تبقى إيجابياً لعدة أشهر بعد التخلص من جرثومة المعدة. ولذلك فهي ليست مفيدة في تأكيد الشفاء بعد العلاج بالصادات ولكنها مفيدة للتشخيص الأولي لعدوى جرثومة المعدة والمسح الوبائي.(2)
اختبار تنفس اليوريا (UBT): وهي طريقة غير مباشرة للكشف عن وجود هذه الجراثيم بناء على قدرتها على إنتاج إنزيم اليورياز، إذ يبتلع المريض اليوريا الموسومة بالكربون 13 أو 14، فإذا كان اليورياز موجوداً في المعدة نتيجة لعدوى جرثومة المعدة فسيتم تقسيم ثاني أكسيد الكربون الموسوم وامتصاصه في الدورة الدموية ومن ثم يمكن التحري عن وجوده بتحليل التنفس باستخدام مقياس الطيف. ويعتبر هذا الاختبار معيارياً من قبل بعض الباحثين فهو غير باضع وغير المكلف مع حساسية ونوعية > 95%، كما أنه الوسيلة المفضلة لتقييم نجاح العلاج بالصادات في الممارسة السريرية، ولكن هناك احتمال حدوث نتائج إيجابية خاطئة عندما يكون هناك فرط نمو للجراثيم المنتجة لليورياز.(2)
اختبار المستضد في البراز (SAT): يعتمد الاختبار على اكتشاف مستضد جرثومة المعدة في البراز إذ تظهر جرثومة المعدة الملتصقة بظهارة المعدة عند الأشخاص المصابين في البراز نتيجة للتساقط الطبيعي للظهارية، وهذا يعني أن الاختبار هو اختبار مباشر للعدوى النشطة مما يمنحه ميزة على الاختبارات المصلية. إنه مقايسة مناعية إنزيمية متوفر بالأشكال متعددة النسيلة ووحيدة النسيلة، ويمكن اعتباره بديلاً لـ UBT في التشخيص الأولي للمرضى الذين يعانون من عسر الهضم الذين لا يحتاجون إلى تنظير فوري. يوصى بالانتظار لمدة 12 أسبوعاً لتأكيد التخلص من جرثومة المعدة بشكل مؤكد. ويتم إضعاف دقته التشخيصية بسبب مثبطات مضخة البروتون ونزيف الجهاز الهضمي، والميزة السلبية الرئيسية مرتبطة بصعوبة التعامل مع البراز.(2)
علاج جرثومة المعدة (Helicobacter pylori)
الخيارات العلاجية الأولى
العلاج الثلاثي مثبطات مضخة البروتون + أموكسيسيلين + كلاريثروميسين
يتكون خيار الخط الأول لعدوى البكتيريا هيليكوباكتر بيلوري علاجاً ثلاثياً قياسياً يتألف من اثنين من الصادات هما كلاريثروميسين وأموكسيسيلين/ميترونيدازول بالمشاركة مع مثبط مضخة البروتون.(6)
كان هذا المزيج أول علاج موصى به على نطاق واسع وحل محل العلاجات الثلاثية الأقل فعالية وتم تقييمه بشكل جيد على مدى سنوات، المحدد الرئيسي لنجاح العلاج بهذه المجموعة هو المعالجة المسبقة لمقاومة الكلاريثروميسين. إن مدة العلاج المثالية هي مسألة خلافية، وكانت الدراسات الأولية تشير غالباً إلى مدة 7 أيام للعلاج وعادة ما يتم اعتماد مدة 14 يوماً من المناطق التي تكون مقاومة الكلاريثروميسين عالية.(1)
يُوصى عادةً بالمعالجة لمدة أطول في بعض البلدان ذات الموارد الجيدة ولكن هناك حاجة إلى مزيد من طرق العلاج الأقصر في المناطق الفقيرة بالموارد، وتجدر الإشارة إلى وجود تقارير عن معدلات شفاء مقبولة عند تطبيق العلاج الثلاثي لمدة أسبوع واحد من العديد من البلدان ولم تتم دراسة وجود فائدة إضافية لفترات العلاج الأطول.(1)
العلاج الرباعي القائم على البزموت
لقد ثبت أن العلاج الرباعي القائم على البزموت له نتائج أفضل فيما يتعلق بعلاج البكتيريا هيليكوباكتر بيلوري ، فالبزموت مسؤول عن عمل أملاح البزموت داخل الجهاز الهضمي العلوي، ويملك البزموت عدة خصائص تساعد في القضاء على جرثومة المعدة من خلال تثبيط مجموعة متنوعة من الإنزيمات وتوليد جزيئات الطاقة ATP ومنع التصاق الجرثيم بالغشاء المخاطي للمعدة، إضافة إلى أن البزموت يعزز إطلاق البروستاجلاندين وعامل نمو الظهارية والبيكربونات والتي تعتبر مواد واقية للغشاء المخاطي كما أنها تساعد في التئام القرحة، ولا توجد تقارير عن مقاومة البزموت حتى الآن.(6)
تم الحصول على نتائج جيدة بنطبيق العلاج الرباعي لمدة 7 أيام على الرغم من وجود مؤيدين للمدة الأطول (10-14 يوماً)، وتتمثل السلبية الرئيسية لهذا العلاج في نظام الجرعات غير الملائم (يجب تناوله أربع مرات يومياً) وفي التأثيرات الجانبية الشائعة (لكن المعتدلة عادةً) والتي قد تضعف التزام المريض بتطبيق العلاج. (1)
العلاجات الرباعية بدون البزموت
هناك تأييد لتطبيق العلاجات الرباعية بدون البزموت ما يعني إضافة ميترونيدازول إلى العلاج الثلاثي (مثبطات مضخة البروتون + أموكسيسيلين + كلاريثروميسين) وقد يؤدي هذا إلى زيادة معدلات القضاء على جرثومة المعدة في حال كانت معدلات مقاومة الميترونيدازول منخفضة أو معتدلة، وغالباً ما يكون المرضى الذين فشل علاجهم لديهم مقاومة مزدوجة لكل من الكلاريثزميسين والميترونيدزول.(1)
العلاج الثلاثي بالليفوفلوكساسين
تم استخدام العلاج الثلاثي (مثبطات مضخة البروتون + أموكسيسيلين + ليفوفلوكساسين) لمدة 10-14 يوماً في خيارات الخط الأول العلاجية عندما تكون مقاومة الليفوفلوكساسين معروفة أو يُفترض أنها منخفضة، ولكن لم يتم دراسة المجموعة على نطاق واسع، وستحد التقارير التي تشير إلى ارتفاع معدلات مقاومة الليفوفلوكساسين في بعض البلدان من فائدة هذا العلاج فيها. ويعتبر العلاج جيد التحمل رغم المخاوف مؤخراً من مخاطر الليفوفلوكساسين التي ترتبط بالمخاطر النادرة لالتهاب الأوتار أو التهاب العضلات الذي يكون أكثر شيوعاً عند كبار السن والذين يعانون من التهاب المفاصل الالتهابي أو القصور الكلوي.(1)
الخيارات العلاجية الثانية أو العلاج الانقاذي
العلاج الرباعي القائم على البزموت والعلاج الثلاثي بالليفوفلوكساسين
تشمل الخيارات العلاجية كخط ثانٍ الأكثر شيوعاً والتي تمت دراستها واستخدامها كل من العلاج الرباعي القياسي القائم على البزموت لمدة 7-14 يوماً وعلاج الليفوفلوكساسين الثلاثي لمدة 10-14 يوماً، وقد ثبت أن كلاهما يحقق معدلات قضاء على جرثومة المعدة أعلى من 80%، ويعتمد الاختيار بين الاثنين على ما إذا كانت هناك معرفة أم لا بمعدلات مقاومة الليفوفلوكساسين، وتوافر الدواء، وخبرة تطبيقه، والالتزام بتطبيقه، والتكلفة.(1)
علاجات الإنقاذ الأخرى
تشمل علاجات الإنقاذ الأخرى التي تم استخدامها العلاج الثلاثي القائم على ريفابوتين، ولكنه أقل فعالية وقد يصل خطر قلة العدلات الكبيرة فيه إلى 1% مما يحد من استخدامه، وعادة ما يتم تجنبه في المناطق التي ينتشر فيها مرض السل بشكل كبير. كما تم استخدام جرعة عالية من مثبطات مضخة البروتون المزدوجة مع الأموكسيسيلين وحققت بعض النجاح، أما العلاجات الرباعية بدون البزموت فهي غير فعالة بشكل عام كعلاجات إنقاذ بسبب مقاومة الكلاريثروميسين والميترونيدازول، ولكن عند تأكيد حساسية الميترونيدازول يمكن استخدام مع الاموكسيلين ومثبطات مضخة البروتون كأحد الخيارات العلاجية الثانية مع نتائج معقولة.(1)
اختبار تقييم نتيجة علاج القضاء على جرثومة المعدة
يجب إجراء تقييم النتائج العلاج في جميع المرضى لأن نتائج نجاح القضاء على جرثومة المعدة متباينة جداً بالرغم من أن هذا قد لا يكون ممكناً على مستوى العالم، ويجب إعطاء أولوية التقييم لأولئك الذين لا يزالون معرضين لخطر الإصابة إذا استمرت الجرثومة مثل أولئك الذين يعالجون من مرض القرحة المعقدة (النزيف أو الانثقاب).(1)
يمكن استخدام الاختبار القائم على الخزعة لتحديد النتيجة بعد علاج القضاء على جرثومة المعدة عندما يكون التنظير مطلوباً (لتقييم التئام قرحة المعدة واستبعاد الأورام مثلاً)، لكن بخلاف ذلك يفضل إجراء الاختبارات غير الباضغة في التقييم. ويجب إجراء اختبارات تنفس اليورياز واختبارات البراز بعد شهر واحد على الأقل من الانتهاء من علاج الجرثومة لتقليل النتائج السلبية الكاذبة، ويجب ألا يأخذ المريض أي صادات حيوية أو مركبات البزموت لمدة شهر على الأقل قبل الاختبار، كما يجب تجنب استخدام مثبطات مضخة البروتون لمدة أسبوع واحد على الأقل ويفضل أسبوعين. وتعتبر الاختبارات المصلية غير مفيد في تقييم نتيجة علاج جرثومة المعدة لأن مستويات الأضداد غالباً ما تستمر لسنوات بعد العلاج. (1)
لقد أدت النتائج غير المثالية في العلاجات الكلاسيكية إلى الحاجة إلى تطوير أدوية علاج جديدة لالتهاب المعدة الناجم عن جرثومة المعدة، وفي عام 2017 قامت منظمة الصحة العالمية بتصنيف جرثومة المعدة ضمن ستة عشر نوعاً من الجراثيم المقاومة للصادات المصنفة على أنها جراثيم ذات أولوية عالية والتي تشكل أكبر تهديد لصحة الإنسان في جميع أنحاء العالم، وذلك استناداً إلى المعدلات العالية لمقاومة الصادات والأدلة الوبائية الحديثة.(6)
المراجع
- World Gastroenterology Organisation Global Guidelines. Helicobacter pylori 2021.
- Abiodun Christopher Jemilohun et al. Helicobacter pylori infection: past, present and future. The Pan African Medical Journal 2016;23:216.
- M. Bagirova, A.M. Allahverdiyev, E.S. Abamor, H. Aliyeva, G. Unal, T.D. Tanalp. An overview of challenges to eradication of H. pylori infection and future prospects. European Review for Medical and Pharmacological Sciences 2017; 21: 2199-2219.
- Aguilera Matos I, Diaz Oliva SE, Escobedo AA, et al. Helicobacter pylori infection in children. BMJ Paediatrics Open 2020;4:1-7.
- Sheila E. Crowe. Helicobacter pylori Infection. Th e new England journal of medicine 2019;380:1158-1165.
- Pop, R.; Tabaran, A.-F.; Ungur, A.P.; Negoescu, A.; Catoi, C. Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles. Pharmaceutics 2022;14:1463.
