يُعتبر التحفيز العميق للدماغ لعلاج مرض باركنسون في تركيا من طرق العلاج الفعالة والناجحة التي تُساعد في تحسين الأعراض، ويمكن أن يُقلل من جرعة الأدوية التي يحتاجها المريض. ومن الممكن أن تكون سبباً في علاج مرض الباركنسون نهائيا.
مقدمة عن مرض الباركنسون ومراحل تطوره
يُعد مرض باركنسون (الشلل الرعاش) ثاني مرض عصبي تنكسي مزمن شيوعاً بعد مرض ألزهايمر الذي يٌعتبر الأول من حيث الشيوع، وهو ذو معدل حدوث مرتفع ويُشاهد بنسبة 1-3% من السكان الذين تتجاوز أعمارهم عن 65 سنة، كما أن معدل حدوثه عند الذكور يفوق الإناث بـ 1.5 مرة.(1)
ربطت الدراسات البحثية النظريات المتعلقة بتفشي مرض الباركنسون بالظروف البيئية والظروف الوراثية، وتقترح هذه النظريات علاقة بين مرض باركنسون بكلٍ من: التفاعلات الكيميائية، السموم العصبية والاستعداد الجيني.(2)
يُعتبر فقدان الخلايا العصبية الدوبامينية للمادة الرمادية في الدماغ السبب الرئيسي في مرض باركنسون، إضافة إلى أن الاختلال في توازن مستويات الدوبامين والأسيتيل كولين في العقد العصبية القاعدية يُؤثر أيضاً على تطور مرض باركنسون.(1)
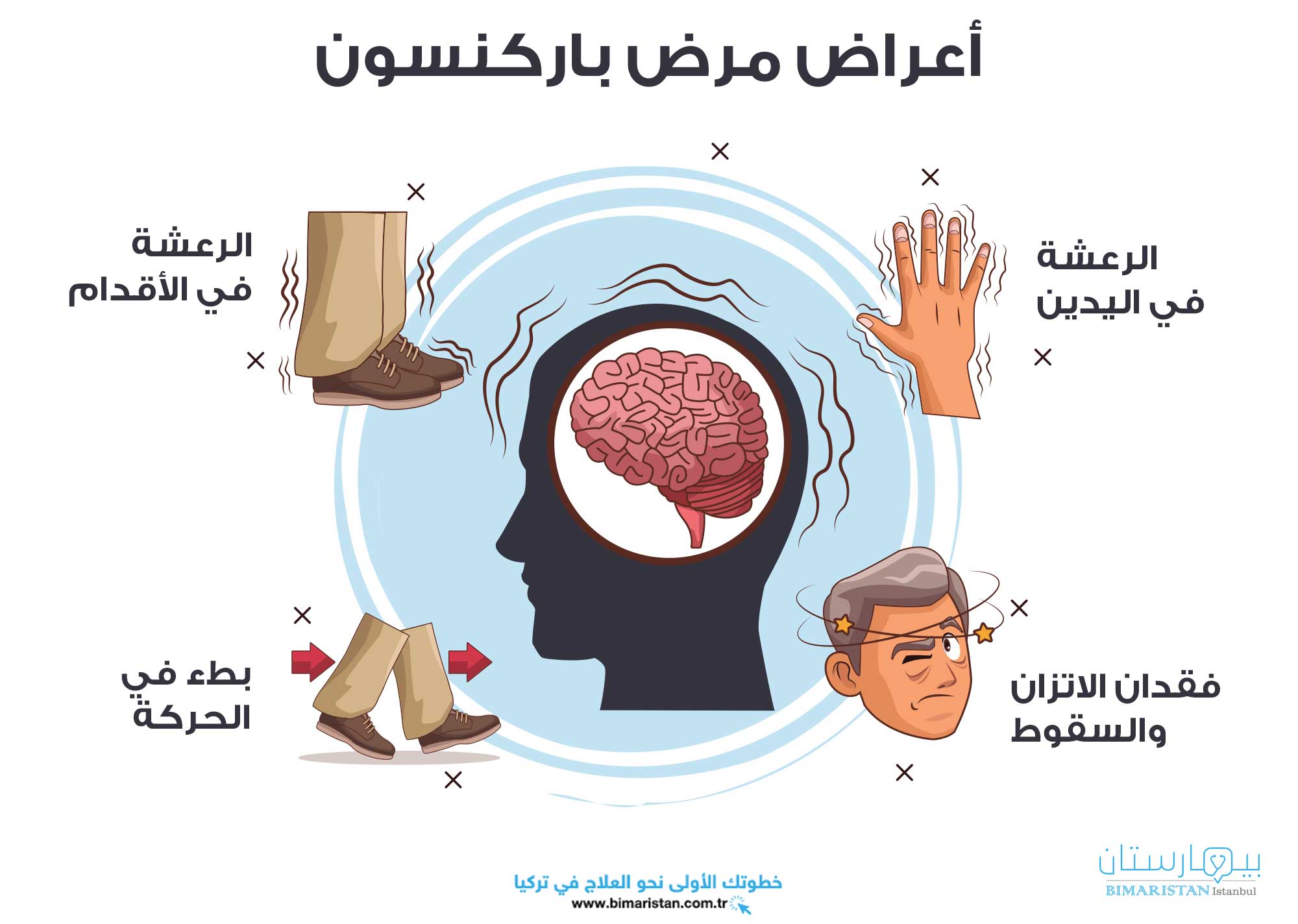
تشمل المظاهر السريرية الرئيسية والمبكرة لمرض باركنسون: بطء أو انعدام القدرة على الحركة، الصلابة (التيبس) ورجفان (الرعاش) الراحة، في حين أن الأعراض المتأخرة في مرض باركنسون هي: انعدام استقرار في الوضعية، اضطرابات المشي والتوازن وهو اضطراب حركي مترقي (يزداد مع الزمن).(1)
يُعتبر التشخيص في مرض باركنسون سريرياً ويمكن إجراؤه باستخدام معايير بنك الدماغ لجمعية داء باركنسون في المملكة المتحدة (UKPDS)، كما أن السمات السريرية الأساسية في مرض باركنسون هي بطء حركة (والذي بدونه لا يمكن وضع التشخيص) ومزيج من الصلابة العضلية، الرجفان (الرعاش) و/أو انعدام الاستقرار في الوضعية (الذي لا يُفسر بحدوث قصور بصري، مخيخي، دهليزي أو في الحس العميق). (3)
عملية التحفيز العميق للدماغ لعلاج مرض باركنسون في تركيا (DBS)
بالرغم من عدم القدرة على تحديد السبب الدقيق في مرض باركنسون، إلا أن الاكتشافات لعلاج المرض كانت تتطور باستمرار، بما أنه لا يوجد علاج شافٍ معروف وتسعى مختلف الطرق لعلاج مرض باركنسون إلى إدارة التظاهرات السريرية وليس منع أو إبطاء تقدم المرض، وتتنوع الأساليب لعلاج مرض باركنسون بين الأدوية، العمليات الجراحية، علاج سلوكي أو مزيج من الطرق التداوي المختلفة.(2)
إن أساليب الجراحة الرئيسية في علاج مرض باركنسون هي: الجراحة الاستئصالية، طريقة التحفيز العميق للدماغ deep brain stimulation parkinson’s، ونقل الخلايا الجنينية المتوسطة إلى الجسم المخطط في الدماغ.(2)
يشكو العديد من مصابي مرض باركنسون المتوسط إلى المتقدم من تدني جودة الحياة لديهم وذلك على الرغم من تطبيق علاج طبي أمثل، ويعود ذلك إلى الاستجابة المتقلبة، خلل حركة مزعج أو الأعراض غير المستجيبة على علاج دواء ليفودوبا (levodopa).(4)
إن الميزة الرئيسية في التحفيز العميق للدماغ لعلاج مرض باركنسون (DBS) تكمن في إمكانية ضبط معايير التحفيز بناء على احتياجات المريض من أجل تطوير النتائج، ويُعتبر التحفيز العميق للدماغ (DBS) المهادي/الوطائي (Thalamic) الأكثر استخداماً للسيطرة على الرجفان كبير المدى لدى المصابين الذين يعانون من الرجفان الأساسي.(4)
يُعتبر التحفيز العميق للدماغ لعلاج مرض باركنسون (DBS) المطبق في منطقة النواة تحت المهاد (STN) أو المطبق داخل الكرة الشاحبة (GPi) هي الأماكن المستهدفة الأكثر شيوعاً للعلاج بواسطة التحفيز العميق للدماغ (DBS) الذين يعانون من الرجفان المُسبب للإعاقة و/أو المضاعفات الحركية المرتبطة مع الليفودوبا.(4)
على الرغم من أن التحفيز العميق للدماغ لعلاج مرض باركنسون (DBS) استراتيجية علاجية مثبتة الفعالية إلا نجاحها يعتمد على الاختيار المناسب للحالات وخبرة ومهارة الجراح بغية زيادة جودة النتائج وتقليل المضاعفات.(4)
إن عمر مريض مرض باركنسون لا يُشكل مؤشراً على نتيجة التحفيز العميق للدماغ (DBS) من حيث الوظيفة الحركية، ويمكن تحديد تحسن مماثل في هذا المجال في كل من الحالات الأصغر سناُ وكبار السن على حد سواء، ومن جهة أخرى فقد تبين أن العمر يُشكل عاملاً متعلقاً بتوقع الآثار المفيدة على جودة حياة مريض مرض باركنسون.(5)
تم تطوير تقنيات التحفيز العميق للدماغ لعلاج مرضى باركنسون (DBS) على مدار الأربعين عاماً الماضية، وقد فتح هذا الباب لتطوير العلاج بهذه الطريقة في مجالات أخرى خارج نطاق اضطراب الحركة مثل الألم والإدراك والحالات النفسية.(6)
يمكن أن يُساعد علاج تحفيز الدماغ الأشخاص المصابين بمرض باركنسون على تحسين أعراض الرجفان والتصلب وبطء الحركة، ويمكن أن يُقلل من الجرعة الدوائية التي يحتاجها المريض لإدارة المرض.
وجد الباحثون الذين تابعوا المصابين بعد الجراحة بطريقة التحفيز العميق للدماغ (DBS) أن العديد منهم يستمرون في التحسن في أعراضهم لعدة سنوات بعد العملية ويمكنهم تناول الطعام واستخدام الحمام وإطعام أنفسهم، كما أن المصابين الذين يعالجون من اضطرابات الحركة بواسطة تقنية التحفيز العميق للدماغ قد يشكون أو لا يشكون من تغيرات في الذاكرة أو التفكير أو الحالة المزاجية.
تُعتبر عملية التحفيز العميق للدماغ لعلاج مرض باركنسون (DBS) قابلة للعكس ولا تؤدي إلى تلف دائم في أنسجة الدماغ، إضافة إلى إمكانية ضبط التحفيز العميق أو برمجته بما يتوافق مع أعراض المريض بطريقة أفضل.
آلية تأثير التحفيز العميق للدماغ لعلاج مرضى باركنسون (DBS)
يقوم التحفيز العميق للدماغ لعلاج مرض باركنسون على تطبيق حقل كهربائي لإحداث التحفيز في الخلية العصبية (وخاصة محور الخلية العصبية) مما يؤدي إلى فتح وإغلاق قنوات الصوديوم في غشاء الخلية العصبية وبالتالي توليد إمكانات العمل والتحكم في تحرير النواقل العصبية، ولا يزال من غير الواضح ما إذا كانت هذه الآلية بالمجمل مثبطة أو محفزة أو ما إذا كانت التأثيرات في الغالب محلية في الخلية أو على مستوى الشبكة العصبية.(6)
هناك أربع نظريات رئيسية لتفسير آلية عمل جهاز تحفيز الدماغ في علاج مرض باركنسون: 1) التثبيط المباشر للفعالية العصبية، 2) الإثارة المباشرة للفعالية العصبية، 3) انقطاع المعلومات ، 4) التصفية المتشابكة العصبية.(6)
على الرغم من أن النظريات الحالية حول آلية عمل التحفيز العميق للدماغ لعلاج مرض باركنسون تركز عموماً على التأثيرات الفورية إلا أن هناك أدلة على أن التحفيز العميق للدماغ قد يؤدي إلى مرونة متشابكة وعصبية، كما أن هناك بعض الأدلة التي تشير إلى أنها قد يؤدي إلى تكوين خلايا عصبية والتشابك العصبي وربما حماية خلايا المخ.(6)
معايير تؤهل المريض لجراحة التحفيز العميق للدماغ في مرض باركنسون (DBS)
تُوصي القواعد الألمانية الحالية بأن المعايير التالية إلزامية للنظر في التحفيز العميق للدماغ لعلاج مرض باركنسون:
- تقلبات حركية بما في ذلك أعراض حساسية ليفودوبا أو بطء الحركة المُحرض بالعلاج.
- الرجفان الذي لا يمكن علاجه بواسطة الدواء.
- تناقص في الأعراض الحركية المحرض بالليفودوبا بنسبة> 33٪ من مقياس التصنيف الموحد في مرض باركنسون (UPDRS)، إذ يمكن تجاهل الرجفان أثناء حساب مقياس التصنيف لأنه قد يكون معند على علاج بالليفودوبا بينما لا يزال يستجيب جيداً في التحفيز العميق للدماغ لعلاج مرض باركنسون.
يُوصى بمعايير قبول أكثر تقييداً لمرضى مرض باركنسون الذين لا تزيد أعمارهم عن 60 عاماً وبوجود تقلبات حركية لمدة لا تزيد عن 3 سنوات.(5)
معايير تجعل المريض غير مؤهل لجراحة التحفيز العميق للدماغ
- الأمراض المرافقة غير المستقرة (مثل إصابة الشرايين التاجية، عدوى إنتانية نشطة، أمراض الأوعية الدموية الدماغية المسببة للعجز، الأورام الخبيثة).
- الاضطرابات السلوكية العصبية والنفسية (مثل الاضطراب الذهاني، الاضطراب ثنائي القطب، الاكتئاب، اضطراب الشخصية الشديد).
- خرف في الحالة النهائية.
- تشخيص غير مؤكد لمرض باركنسون.
- ظهور ضخامة كبيرة في بطينات دماغية أو ضمور دماغي في التصوير بالرنين المغناطيسي.
- أعراض محورية شديدة مقاومة للعلاج بليفودوبا (عسر التلفظ، عسر البلع، عدم استقرار الوضع أو اضطرابات المشي).
- عدم وجود إعاقة وظيفية.
- عدم القدرة على تقديم موافقة مستنيرة على العمل الجراحي.
- الصعوبات الاجتماعية أو الجغرافية في الوصول إلى المستشفى لزيارات المتابعة وبرمجة جهاز التحفيز.
- الدعم الاجتماعي غير الكافي من الأسرة أو مقدمي الرعاية.(7)
الإجراء الجراحي المطبق في التحفيز العميق للدماغ لعلاج مرض باركنسون
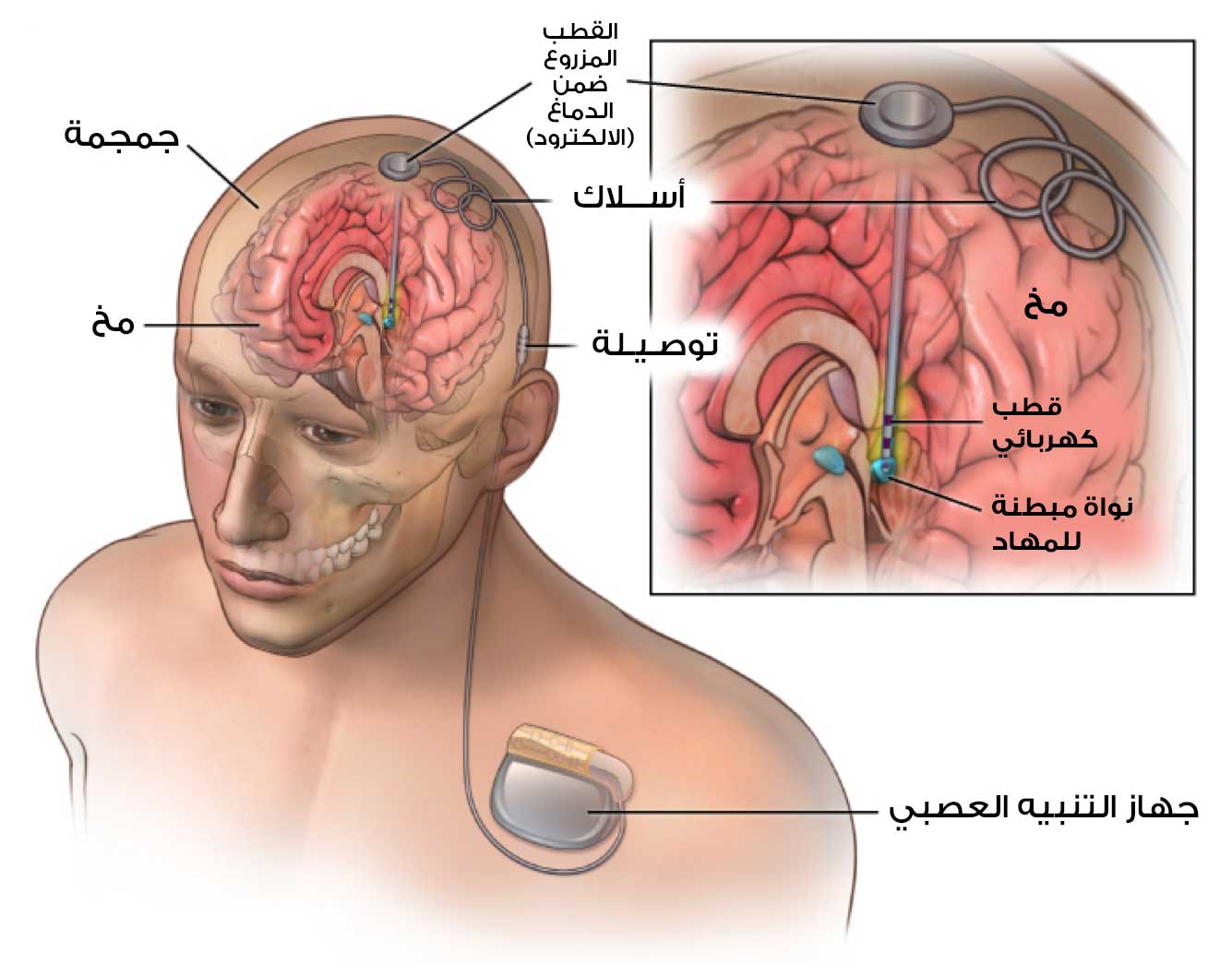
يجب إجراء مسح للوضع التشريحي لدماغ للمريض بواسطة التصوير بالرنين المغناطيسي كشرط أساسي لتحديد النقطة المسهدفة ومسار وضع القطب المستخدم في التحفيز العميق للدماغ لعلاج مرض باركنسون.(5)
بعد شق الجلد وحفر ثقب يمنح مدخلاً للمسار المخطط له يتم إدخال أقطاب كهربائية دقيقة إما خطوة بخطوة (كل خطوة 0.5-1 مم) أو يتم توجيهها باستمرار على طول المسار المخطط له لإجراء التحفيز العميق.(5)
قد يساعد إجراء التسجيل بالأقطاب الدقيقة (MER) في تحديد المناطق المستهدفة من خلال أنماط نشاط مميزة يتم تحديدها عبر الإطلاق العفوي، التفريغ المفاجئ، وتغيرات النشاط بسبب الحركة أو المنبهات الحسية وبالتالي قد يزيد من دقة الوضع النهائي للقطب إجراء التحفيز العميق.(5)
يمكن للعتبات المنخفضة نسبياً من الآثار الجانبية المميزة لعملية التحفيز العميق للدماغ لعلاج مرض باركنسون -مثل حدوث التقلص الشبيه بالكزاز بسبب تحفيز المحفظة الداخلية- أن تساعد في تحديد القرب من المناطق التشريحية المسؤولة عن ظهور الآثار الجانبية في التحفيز العميق للدماغ، وبالمقابل تُشير العتبات المنخفضة للتأثيرات المفيدة دون تطور الآثار الجانبية في التحفيز العميق للدماغ لعلاج مرض باركنسون إلى المكان المفضل لوضع القطب، وبمجرد تحديد الهدف الأمثل يتم إدخال القطب التحفيز العميق للدماغ بشكل نهائي.(5)
يمكن للمرضى الذين يخضعون لجراحة التحفيز العميق للدماغ لعلاج باركنسون في حالة الاستيقاظ أن يتمتعوا بتحسنٍ أسرع ونتائج أفضل خاصة فيما يتعلق بأعراض مثل عسر التلفظ إذ يمكن بسهولة اكتشافها خلال التحفيز الاختباري أثناء الجراحة مما يسمح باتخاذ قرارات أفضل بشأن وضع القطب النهائي.(5)
تتضمن العملية وضع ثلاثة أجزاء في الجسم يتم زرعها كلها تحت الجلد، فالجزء الأول هو الأقطاب الكهربائية التي تدخل الدماغ، والجزء الثاني هو حزمة البطارية (أو المولد) والذي توضع في الصدر أسفل عظمة الترقوة مباشرة، أما الجزء الثالث فهو الأسلاك التي تربط الأقطاب بالبطارية.
تختلف استجابة المرضى بعد التحفيز العميق للدماغ من مريض لآخر، وغالبا ما تتم ملاحظة النتائج بعد وقت قصير من البرمجة الأولية من أجل التحفيز، وقد يستغرق الأمر عدة زيارات لضبط إعدادات التحفيز العميق لتحقيق أقصى فائدة، ويمكن تحديثها باستمرار مع تغير الأعراض بمرور الوقت.
تستغرق عملية زراعة الأقطاب الكهربائية بضع ساعات، ويتم الزرع على مرحلتين الأولى يتم فيها وضع الأقطاب في الدماغ ويحتاج المريض إلى قضاء ليلة في المستشفى، أما خلال المرحلة الثانية فيتم زرع البطارية وعادة ما يعود المريض إلى المنزل في نفس اليوم.
بناء على حالة المريض قد يكون من الممكن تقليل الأدوية، ومع ذلك يكون التحفيز العميق للدماغ مفيداً للغاية عند استخدامه مع الأدوية والعلاجات الأخرى لأنه سيكون من الممكن خفض جرعات الدواء وتقليل الآثار الجانبية مع استمرار الحصول على نفس الفوائد.
مضاعفات أو مخاطر التحفيز العميق للدماغ لعلاج مرض باركنسون
يمكن أن ينتج عن جراحة التحفيز العميق للدماغ لعلاج مرض باركنسون مخاطر ومضاعفات مرتبطة بالجراحة، ويُعبتر النزف داخل الدماغ الخطر الجراحي الرئيسي وتبلغ نسبة حدوثه حوالي 1-2% بما في ذلك النزف البسيط، كما تُعتبر النوبات خطراً لأي علاج جراحي فوق الخيمة وتحدث نسبة 1%.(6)
يمكن أن تحدث مضاعفات دوائية- كاللتي تتلو أي علاج جراحي- بعد إجراء التحفيز العميق للدماغ بنسبة تقل عن 2%، وتتضمن: الخثار الوريدي العميق، التهاب الأوردة، ذات الرئة، انتانات المسالك البولية والصمة الرئوية.(5)
ترتبط بعض المضاعفات مباشرة بجهاز التحفيز العميق للدماغ نفسه، ويمكن أن تشمل هجرة الرصاص والكسر بنسبة 2-3% وحدوث انتان في الجهاز بنسبة 3-8%، كما قد تحدث أيضاً مضاعفات من التنبيه الكهربائي في التحفيز العميق للدماغ حسب المكان المستهدف في التحفيز العميق والموقع التشريحي للقطب، وتتراوح هذه المضاعفات من عجز الأعصاب القحفية والأعراض الحركية إلى الاضطرابات النفسية والاضطرابات اللاإرادية.(6)
يبلغ معدل الوفيات من جراحة التحفيز العميق للدماغ لعلاج مرض الباركنسون حوالي 0.4% ويرتبط غالباً باحتشاء عضلة القلب بعد الجراحة والصمة الرئوية.(6)
تأهيل المريض بعد التحفيز العميق للدماغ
يجب أن تهدف برامج إعادة التأهيل إلى علاج المشاكل الوظيفية إضافة إلى منع المشاكل التي ستنشأ لاحقاً، وقد يساعد البدء ببرنامج التأهيل بالتزامن مع علاج المرض من المراحل المبكرة في تجنب مثل هذه المشاكل التي تؤدي إلى الاعتماد على الآخرين، الخمول، العزلة الاجتماعية، وانخفاض نوعية الحياة.(8)
مرحلة إعادة التأهيل قبل عملية التحفيز العميق في تركيا
تتمثل الخطوة الأولى في إجراء تقييم قبل عملية جراحة بأالتحفيز العميق للدماغ في إبلاغ المريض وعائلته بالمضاعفات المحتملة، كما يتضمن إجراء تقييم لكل من: التوازن، الحركة، الرجفان، الصلابة، التناسق، الكلام، وظائف اليد، أنشطة الحياة اليومية، البيئة المحيطة، واختيار الجهاز المساعد للحركة، ويهدف برنامج إعادة التأهيل في مرحلة ما قبل جراحة التحفيز العميق للدماغ إلى:
- تحسين وظيفة الجهاز التنفسي والوقاية من الاختلاطات
- تقليل الصلابة
- تقليل الألم
- الحفاظ على الاستقلال عن الآخرين
- تحسين المرونة
- تحسين المشي
- زيادة مستوى التنسيق والتوازن الحركي الإجمالي
- التثقيف والتوجيه باحتياجات تقديم الرعاية.(8)
مرحلة إعادة التأهيل ما بعد الجراحة
يتم تقييم مرضى التحفيز في أول 24 ساعة بعد ضبط البطارية، ويعاني مرضى داء باركنسون من فشل تنفسي بسبب وضعية الانحناء والحداب والصلابة، ويبدأ العلاج الفيزيائي بتمارين الجهاز التنفسي وتمديد الصدر وتمارين محاذاة الوضعية والتي تُعد فعالة في زيادة قدرة الجهاز التنفسي.(8)
يجب تعليم المريض الوضع الصحيح من أجل الحد من اضطرابات الوضع ومشاكل التوازن، كما يجب تطبيق تمارين التمديد الوضعي وحركات دوران الجذع.(8)
بهدف منع السقوط يجب توجيه المريض للدوران حول قوس كبير عن طريق وضع القدم بشكل صحيح وزيادة المنبهات البصرية واللفظية أثناء المشي، كما يجب تعليم المشي بإيقاع والمشي السليم مع دوران الذراع لمنع التجمد، ومن المهم إعطاء فترات راحة متكررة أثناء التمارين وتجنب الإرهاق المفرط.(8)
توصيات للمريض وعائلته بعد التحفيز العميق للدماغ
يجب على المرضى بعد الجراحة الامتناع عن مجموعة النشاطات التالية:
- التدليك والتمارين المفرطة للرقبة والعنق.
- القيام بأنشطة الأطراف العلوية فوق مستوى الرأس.
- حمل ثقل يتجاوز 3-4 كغ في الشهر الأول.
- استخدام بعض الأجهزة الطبية مثل التصوير بالرنين المغناطيسي.
- المرور عبر البوابات الكهرومغناطيسية (أجهزة الكشف في المطارات).
- التعرض لموجات الاتصال اللاسلكي لأنها تشكل خطورة على البطارية.
- التعرض للآلات الصناعية ذات الطاقة العالية.
- يمكن القيام بأنشطة رياضية بسيطة خاصة تلك التي لا تشكل خطورة جسدية على الاصطدام ودون ملامسة منطقة العنق أو جهاز تحفيز الأعصاب.(8)
سعر عملية التحفيز العميق للدماغ لعلاج مرض باركنسون في تركيا
يختلف سعر عملية التحفيز العميق للدماغ لعلاج مرض باركنسون في تركيا حسب نوع المركز الذي سيتم فيه اجراء العملية حيث تبلغ التكلفة في مشفى الدولة حوالي 22 ألف دولار أمريكي بينما تكون التكلفة في المشافي الخاصة حوالي 27 ألف دولار بينما قد تصل إلى 33 ألف دولار أمريكي في أفضل مركز لعلاج التحفيز العميق.
المراجع
- Ibrahimoğlu and Akyol. Early-term results of deep brain stimulation in Parkinson’s disease: a case report. Cukurova Medical Journal 2019;44:1499-1505.
- Lee et al. A review on Parkinson’s disease treatment. Neuroimmunology and Neuroinflammation 2021;8:222-244.
- Esmail S. The Diagnosis and Management of Parkinson’s Disease. Sch J Appl Sci Res 2018; 1: 13-19.
- Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 2020;91:795-808.
- CJ Hartmann, S Fliegen et al. An update on best practice of deep brain stimulation in Parkinson’s disease. Therapeutic Advances in Neurological Disorders 2019;12:1-20.
- Lee et al. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg 2019;131:333-342.
- Brandão P et al. DBS in Parkinson’s disease: therapeutic decisions. Arq Neuropsiquiatr 2018;76(6):411-420.
- Ünal A, Altug F. Clinical Experience from Turkey in Rehabilitation of Parkinson’s Disease after Deep Brain Stimulation: What are we Doing?. Physiother Rehabil 2017;2:142.
